What is an HA tag? An HA tag is derived from the human influenza C-terminal hemagglutinin (HA)-tagged recombinant molecule corresponding ...
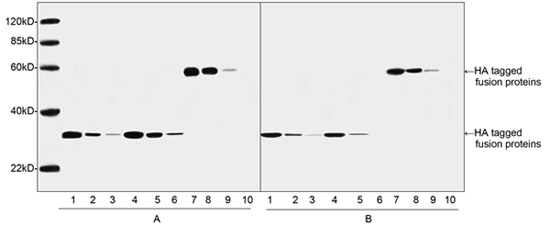

What is an HA tag? An HA tag is derived from the human influenza C-terminal hemagglutinin (HA)-tagged recombinant molecule corresponding ...
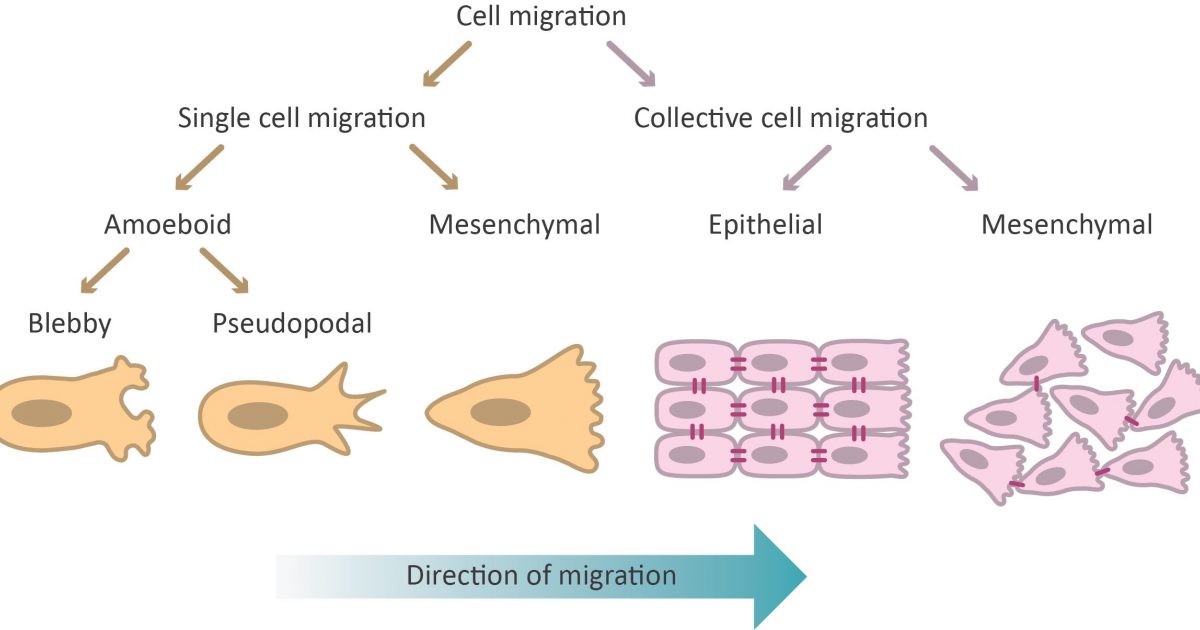
What is cell migration? What is cell migration and what is its physiological relevance? Cell migration is the directed movement ...
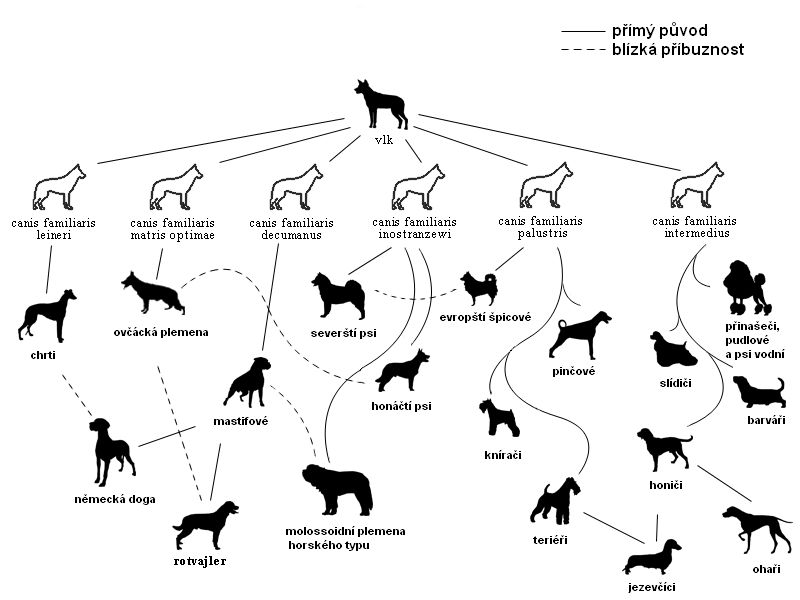
Abstract Recent studies have shown that domestic Canis lupus familiaris Recombinants are poorly susceptible to visual illusions, suggesting that the ...