What is cell migration?
What is cell migration and what is its physiological relevance?
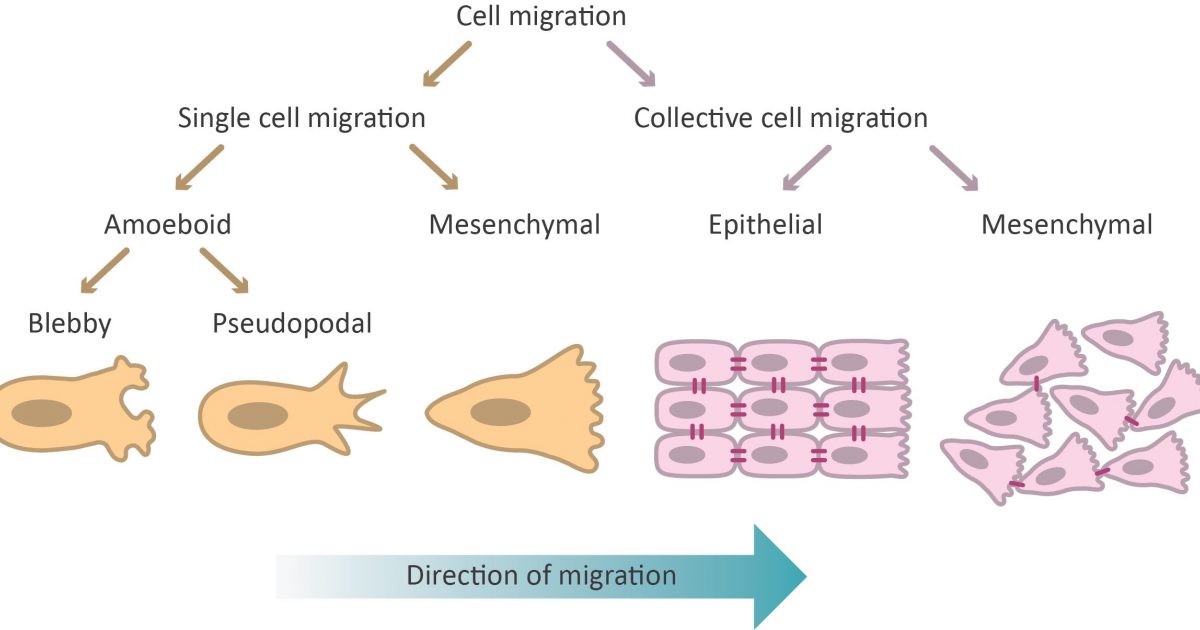
Cell migration is the directed movement of a single cell or a group of cells in response to chemical and/or mechanical signals. It is a fundamental cellular process that occurs throughout life, beginning during embryonic development and continuing until death, and can sometimes contribute to pathogenic states in disease.
In a developing embryo, cell migration is the driving factor for various morphogenetic events. For example, during gastrulation in very early embryos, groups of cells migrate as sheets to form the three germ layers. Subsequently, cells from the germ layers migrate to various destinations, where they specialize into distinct cell populations that form various tissues or organs in the embryo.
In adult organisms, cell migration Recombinants occurs during vital cellular processes, such as tissue renewal and repair, in which old or damaged cells are replaced by the migration of newly formed cells from underlying tissue layers. Such events are essential to maintain tissue integrity and homeostasis. Cell migration also plays a role in mediating immune responses during infections, in which phagocytic cells, such as neutrophils circulating in the bloodstream, migrate to infected tissues and destroy invading pathogens.
Although, on the one hand, cell migration is vital to maintain tissue health and homeostasis, on the other hand, undesirable migratory events are causative factors for a number of pathological states, such as inflammatory diseases, cancers, etc. Therefore, cell migration has to be a strictly controlled process -both in time and in space- to maintain a homeostatic state in an organism.
Cell migration as a cyclic process
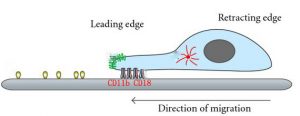
The migration of a single cell or a group of cells is considered to be a cyclical process, involving polarization of cells in response to migratory signals, an extension of filopodial or lamellipodial protuberances, formation of adhesions between the cell and the underlying matrix, and the pushing of cells on the adhesions as a result of the traction forces generated by the adhesions.
1. Polarization of migratory cells:
The first step in directional migration is cell polarization, during which the front and back of the cell become different in structure and molecular composition. The Rho family of GTPases, mainly Rac, Cdc42, and Rho, is one of the key regulators of cell polarization, each showing localized activity in cells. While Rac and Cdc42 show localized activity at the leading edge, active Rho accumulates at the sides and rear of the cell.
Cdc42 also regulates the MTOC to locate it in front of the nucleus, closer to the leading edge. This is mediated by the PAR6 effector of Cdc42, which forms the “PAR polarity complex together with PAR3 and aPKC; aPKC binds to tubulin subunits on newly formed microtubules and anchors them at the leading edge. The assembly of microtubules towards the leading edge facilitates the delivery of cargo (membrane and proteins) that are used in the formation of cell protuberances.
2. Prominence extension:
A polarized cell begins to produce actin-based protrusions on its leading-edge, such as lamellipodia or filopodia. Lamellipodia form as branching dendritic networks of actin filaments and can therefore push along a broader stretch of the membrane. Filopodia, on the other hand, are formed as parallel bundles of actin filaments and have functioned primarily in sensing the physical properties of the extracellular environment. The molecular mechanisms that drive the formation of these bumps are different; lamellipodia are made up of the proteins of the Arp2/3 complex, which attach to the sides of preexisting filaments and initiate the assembly of newer filaments that branch off from the main filament.
The activity of the Arp2/3 complex is regulated by the Wasp/Wave family of proteins, which in turn are regulated by Rho GTPases. Assembly of the filopodium occurs via a treadmill mechanism, in which actin monomers are added at one end (spiked) and disassembled at the other end (pointed) in a stable state. Various actin-binding proteins such as Ena/Vasp, fascin, ADF/cofilin, and guard proteins regulate the rate of filopodial actin assembly.
3. Adhesion formation:
The extension of the bulges is accompanied by the assembly of molecular structures called focal adhesions that connect the actin cytoskeleton with the extracellular matrix (ECM). This is often initiated by interactions between ECM components (ligands) and receptors (primarily integrins) on cell surfaces, which then activate distinct intracellular signalling pathways and cause the sequential recruitment of various signalling, regulatory, and cellular proteins. scaffolding to focal concentration sites adhesions.
Focal adhesions serve two important roles at the leading edge: as traction sites against which cells generate tensile forces to push themselves forward, and as mechanosensory that convey information about the physical properties of the matrix to the interior of the cell. Tensile forces are generated due to the interaction of myosin bundles with actin filaments anchored at focal adhesion sites and contractile activity between the two molecular assemblies.
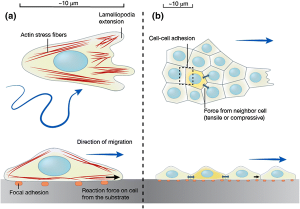
The migratory capacity of cells depends on the strength of focal adhesions, which is influenced by factors such as ligand density, receptor density, and ligand-receptor affinity. For example, rapidly migrating cells have very few groups of integrins, and therefore these cells form very few submicroscopic adhesions. Cells with evenly distributed groups of integrins form smaller adhesions called focal complexes that stabilize the bulges but can also easily dissociate, leading to efficient migration. On the other hand, cells with mature focal adhesions are highly adherent and therefore non-migratory or slow-moving.
4. Adhesion disassembly:
Adhesion disassembly occurs both at the leading edge and at the back of migrating cells. At the leading edge, older adhesions at the base of the bulge often disintegrate; however, some of them do not and instead develop into more mature molecular assemblies. The disassembly of adhesions at the front is regulated by kinases such as =FAK and Src, as well as by phosphatases.
Several studies in this area have led to a model for the Src/FAK-mediated signalling pathway, in which active forms of these kinases lead to activation of Rac and Erk. The final response is the renewal of adhesions in response to activation signals. Adhesion renewal at the rear is essential for tail retraction and forward protrusion of cells and is primarily regulated by myosin II-dependent actin filament contractility.